Electromagnetism is all about how charges affect each other and how the interaction of electrical and magnetic fields induce the flow of charge. It is still the movement of charge, but in this context, it is the movement of a charge through a circuit. As electrons move through a circuit they induce a magnetic field. Conversely, a moving magnetic field will induce the movement of electrons through a circuit. It is the principle behind how we generate most of our electrical energy today through the burning of coal or gas, nuclear power generators, wind turbines, or hydro-power. All rely on spinning coils of copper wire inside a magnetic field to induce that flow of charge. The important point here is that there needs to be a circuit for a current to occur. We outline what a circuit is further below in the section, Make a circuit, create a current.
Hans Christian Oersted, a Danish physicist and chemist, was considered the first to demonstrate the connection between electricity and magnetism. His 1820 experiment used an electric current to deflect a magnetic compass needle. Before this revelation, electricity and magnetism were considered distinct phenomena.
It was known that electricity could produce heat and light. But what Oersted showed was that a flow of charge produces a magnetic field and that we could use electricity to make things turn.
Starting about a decade after Oersted’s discovery, Michael Faraday built on Oersted’s research by working out that while the flow of charge induces a magnetic field, a changing magnetic field will also induce a flow of charge, which he turned into mechanical motion and the world’s first motor, albeit a crude and rather impractical one, but nonetheless it paved the way for the modern electric motor and, soon after, the knowledge to turn that mechanical motion into electricity, which is the way we still generate most of our electricity today. Students can replicate Faraday’s simple electric motor in Activity 9, Electric motors: Spinning wire.
Key Point: a charge affects the electrical field;a moving charge affects the magnetic field
Finding the electron
About 70 years after Oersted’s discovery, a scientist named J. J. Thomson used the effect of electrical and magnetic fields to determine the existence of the electron – and that atoms were not the smallest units of matter.
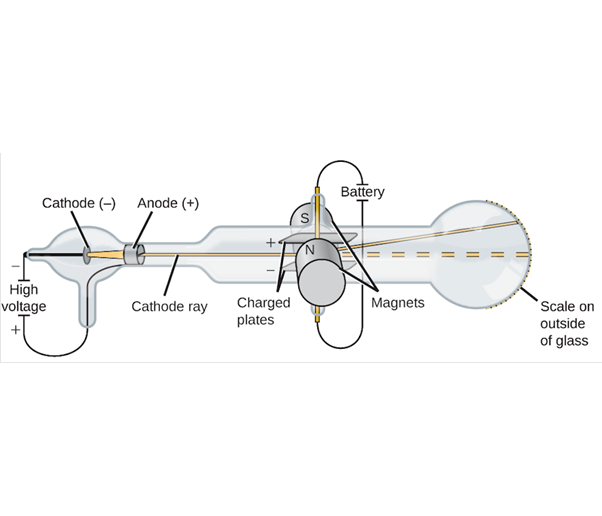
A diagram of J.J. Thomson’s cathode ray tube. Thomson used a high voltage (lots of electric force) applied across two electrodes: one the cathode (with a negative charge), the other an anode (with a positive charge). The ray or beam of electrons originates at the cathode where it travels in a straight line to the end of the vacuum tube and interacts with a chemical substance that glows where the particle beam hits. Thomson set up a charged set of metal plates in the middle of the tube and opposing magnets on each side. Thomson found the particle beam was deflected away from the negatively-charged electric plate, and towards the positively-charged electric plate. Image from Openstax, CC BY 4.0
Further research determined the size of the particles we now call electrons and scientists realized it was tiny – about 1000 times lighter than the smallest atom, which explained why they could pass right through materials and travel through circuit wires. Thompson used this understanding to develop a new model of the atom, which as noted above, was wrong, but the discovery of the electron paved the way for others to develop the nuclear models that we now consider to be correct. Our most recent understanding is based on the electron cloud model. See Figure 1.
References:
Britannica: Rutherford’s nuclear model
Khan Academy: electronic structure of atoms
Make a circuit, create a current
Electrons – the charged particles – can only move when there is a continuous (closed) circuit for the electrons to flow through. Any break in that circuit and the current will not flow. An effective circuit needs a power or energy source, in our case that is a battery. The battery is a source of electrons (from the negative terminal) and the force that pushes the electron through a circuit once you have a closed or complete circuit. In a closed circuit, the positive terminal of the battery will also pull electrons through a circuit because of the attractive force between the positive and negative charges. Without the source of electrical energy, electrons just move around randomly. When the energy source is present and a closed circuit exists, the electrons are pushed (and pulled) in one direction. But remember it is only the electrons that move through the circuit. It is this movement or flow of electrons that enables the generation of electrical energy to power our lights, computers, or any item that runs on electricity. The protons – or positive charged particles – are fixed and cannot move. The energy stored in the battery is chemical energy. When the battery is connected to a circuit, the chemical energy is transformed into electrical energy that we use to provide light and run our household appliances.

Figure 2. Image of a closed electrical circuit.
The Victorian State government Department of Education provide further insight into students’ perception of circuits here
Graphite circuits
Graphite is a form of carbon. In fact, so are diamond and charcoal. These different forms are called allotropes. They are all carbon, but the way the carbon atoms are arranged is different. For example, diamond has its carbon atoms arranged so that each atom is strongly bound to four other carbon atoms. This means there are no spare electrons and it can’t conduct electricity. It also makes it extremely hard. Graphite has carbon atoms arranged in layers and each atom is only bound to three other atoms leaving a spare electron floating around. This make is soft and a good conductor. Your pencil lead is graphite and it works because its layers are loosely bound which is why the graphite comes off easily onto your paper. You can test the conductivity of graphite in Activity 10, Build a graphite circuit.
For more information on FLEET’s research with graphene, see this story, I can’t believe it’s not graphene.
A single layer of graphite is called graphene, which is highly conductive and incredibly strong. By weight, it is about 200 times stronger than steel. As a 2D material with electronic properties, FLEET conducts research on graphene to examine its potential use in energy-efficient transistors and circuits. Nanowerk has put together a nice article on the structure and potential uses of graphene.
Figure 3. A single layer of carbon atoms is known as graphene.
Piezo-electricity and wearable electronics
Piezoelectricity is the conversion of mechanical pressure into electrical energy. The source of piezoelectricity comes from crystals that have a certain structure. When you compress thin slices of these crystals their atoms rearrange in a way that puts a positive charge on one side of the crystal and a negative on the other. You can hook up a wire to the positive side and a wire to the negative side to make a circuit and the difference in charge on each side of the crystal can drive a current through the circuit. Conversely, if you put a current through a piezoelectric material it will cause a distortion in the crystal, which the basis of how speakers work.
See a great explanation on piezoelectrics in this TedX video – https://youtu.be/YEJ2qryXcIQ
Quartz is a natural piezoelectric crystal, but researchers have developed a range of artificial crystals that are more effective. FLEET has helped develop a new type of ultra-efficient nano-thin piezoelectric material that could advance self-powered electronics, wearable technologies and even deliver pacemakers powered by heart beats. The novel material is printable on machines similar to those that print newspaper sheets, it is 100,000 times thinner than a human hair and 800% more efficient than other piezoelectric materials based on similar non-toxic materials. See FLEET News