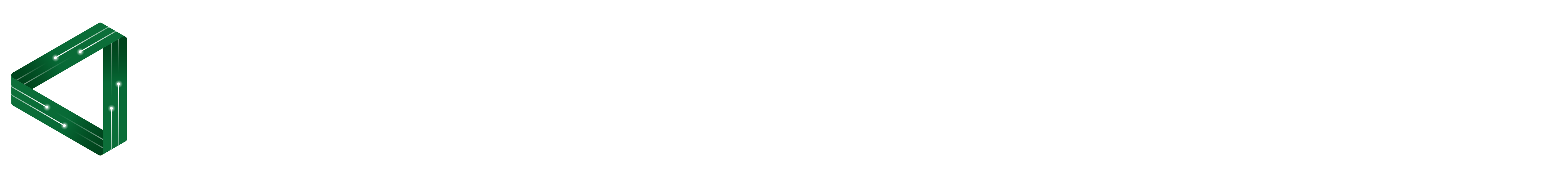
This is a simple experiment that creates a circuit with steel wool and shows the fiery potential of resistance. The steel wool does actually catch fire so make sure you do it safely.
Download activity as a pdf
Learning intentions
Students get to generate inquiry questions about electrical circuits. The activity will help students recognize the need for a complete circuit to allow the flow of electricity and support their investigation into the differences between electrical conductors and insulators.
Hypothesis
You have steel wool made up of fine strands of mostly iron, which is a good conductor of electricity. A single strand of steel wool is really thin and actually quite long by the time you unravel it from the steel wool pad. What do you predict will happen when you place a 9V battery to the steel wool and create a circuit? What is your reasoning for this.
Materials
- steel wool
- 9V battery
- heat proof dish
- Copper wire (1mm-2mm diameter)
**Use nothing but a 9V battery to set steel wool on fire. Adult supervision required.
Method
- Put the steel wool into your heat proof dish.
- Pull on the steel wool to spread it out a bit.
- Touch the terminals (+ and – ) of the 9V battery to the steel wool.
And then it should burn, burn, burn, in a steely fire, in a steely fire…
Try the experiment with your copper wire. Cut a short section (about 10cm) and connect one end to the positive terminal of the battery and the other to the negative terminal. Feel the wire, but do not leave it connected for longer than about 10 seconds. Does it get hot – even warm?
*Note. If you cannot source the copper wire a large metal paper clip can be unfolded and bent so the two ends can touch the battery terminal.
Results
The steel wool should catch alight. Can you explain what is happening? Why does the steel wool catch on fire? Think about resistance. See Figure 1 and 2 below.
The copper wire might get warm, but it will not catch alight. What is the difference between the steel wool and the copper? Again, think resistance.
What is happening?
The fiery response is all about resistance, which we know is futile.
In this experiment, we have created a circuit with the steel wool or copper wire and 9V battery.
Steel wool is mostly iron, which is a good conductor (but not the best). By touching the battery to the steel wool you have created an effective circuit between the battery’s positive and negative battery terminals that allow energy (electrons) to flow from the negative terminal, through the steel wool and into the positive terminal. When electrons flow through a circuit, you generate an electric current. But as the electrons flow through our circuit they bump into and interact with the iron atoms in the steel wool strands and cause them to vibrate and generate heat. That is, as the electrons hit other atoms in the circuit material, the electrons transfer some of their energy into kinetic (movement) energy and produce heat. This process is called resistance. Rub your hands together really fast. Do your hands feel warm? They should this is kinetic energy from the friction between your moving hands being transformed into heat energy.
The intense heat caused by iron’s resistance allows for iron to react with oxygen in the air and catch on fire. After the first bit of steel wool catches on fire, there is a chain reaction that burns the rest of it.
Even the best conductors have resistance, but the atomic structure of some materials means there are more collisions than in others. More collisions mean greater resistance and more heat generated.
And a thin wire will have greater resistance than a thick wire. And the longer the wire, the greater the resistance. The wire in steel wool is really thin and really long.
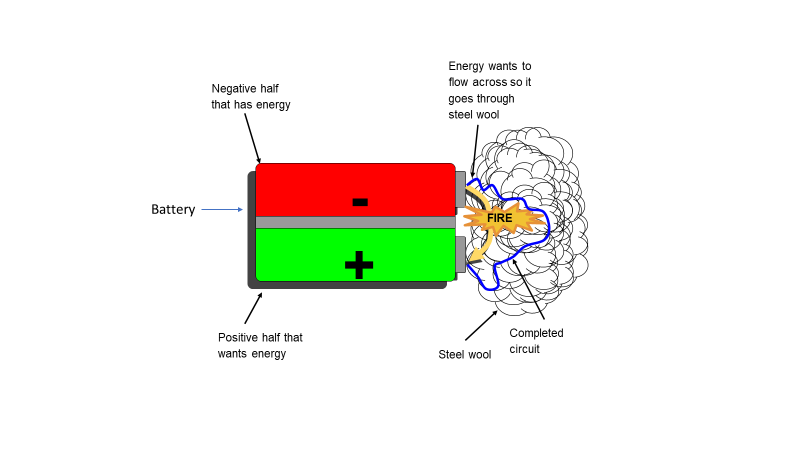
Figure 1. In a fully charged battery, the negative half of a battery is where all the electrons are stored. The other half stores positively charged ions. When you create a circuit between the two halves of the battery, the negative electrons will want to move through the circuit to the positive terminal. The resistance the electrons meet on their journey through the extremely thin steel wool circuit create sufficient heat to cause the thin wool wire to catch fire. Image by Tristian Fuhrer.
In real circuits, we tend to use copper because it has lower resistance than steel and is therefore a better conductor. The lower conductivity and higher resistance of the iron compared to copper is why the steel wool gets hot and catches on fire. Which is another reason we use copper in circuit wires – less likely to burn the house down.
Copper is a better conductor than steel and therefore has lower resistance. The copper wire you used is also a lot thicker than the steel wool so the copper wire will have a lot lower resistance than the steel wool and the electrons will lose a lot less of their energy as heat and only get warm rather than catch on fire.
Sometimes we want to generate heat and therefore light from a circuit (really hot things glow and give off light – for example the Sun). Think of the old-fashioned tungsten filament lightbulb that generates light by using the electrical current to heat up the thin metal filament inside the bulb to produce light.
Some materials are called insulators that effectively prevent any current flowing through a circuit. Try stick a 9V battery on torn up strips of plastic, or wood shavings. Do you start a fire? Do the plastic and wood even get hot? Their atomic structure makes them effective insulators because they prevent any electron flow. Why are all electrical cords plastic-coated?
Electrons: Steel and copper are good conductors because their atoms will easily donate electrons. That is, they have mobile electrons that are not strongly bound to the atom. When a force is applied such as that from a battery, the mobile electrons will begin to flow through the circuits. They are pulled toward the positive terminal (negative is attracted to positive) and pushed by the negative terminal (negative and negative repel). Hence, it is not just the electrons from the battery that are pushed through the circuit. It is the mobile electrons in the wire that move and generate a current. Insulators typically have their electrons strongly bound to their atoms and it takes loads more energy (force) to get them to leave their atoms and move through a material. This another reason insulators have higher levels of resistance.
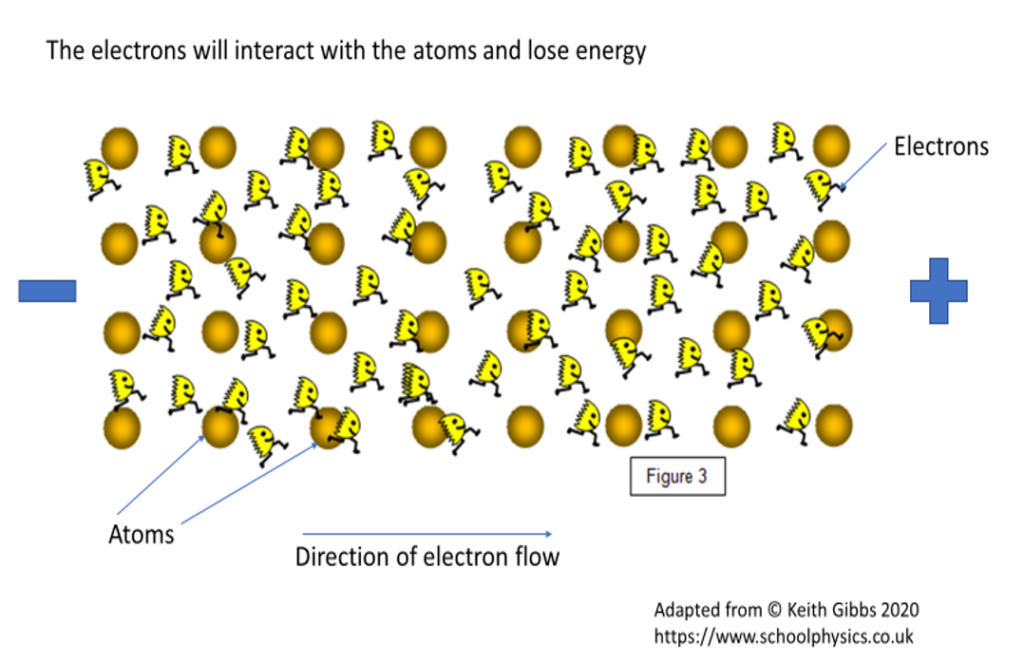
Figure 2. As electrons flow through a circuit they interact with the atoms in the material the circuit is made of and transfer some of their kinetic energy into heat energy.
Students can learn more about circuits in FLEET School’s resource, Conductors, insulators and electricity.
Resistance is futile (or not!) FLEET researchers are working to develop novel materials that can conduct electricity with zero resistance. Why? Because resistance and the heat it generates is wasted energy. If an electron could flow through a material without resistance, all its energy would be used to do the work we want it for, for example, to power all our electrical devices. With zero resistance, no energy would be lost (wasted) as heat. This could help cut our consumption of electricity and production of greenhouse gases. Learn more about FLEET’s research here.
Extended thinking
Student can play around with Ohm’s law and determine the resistance of different circuits in FLEET Schools, Graphite circuit activity.
Back to FLEET Schools or All Home Science activities.