You can be a water bender. All you need is a balloon (and a good head of hair).
Download pdf of Water Bender activity
Learning Intentions
Students get to think, observe and learn about the difference between insulators and conductors and how charged particles (electrons and protons) function to generate static electricity.
Materials
- A dry latex balloon (if you don’t have a balloon you can use a dry plastic comb, a section of PVC pipe, or plastic pen)
- A water tap (or a bottle with a small hole cut in the bottom which allows the water to drizzle out at a slow and steady speed)
- head of hair (or towel)
Before the experiment
You will do two things in this experiment. First you will rub an inflated balloon against your hair. If you don’t have any hair use a towel or the nearest person to you with hair – borrow their head, but ask nicely. Second you will hold that balloon that you rubbed against a head of hair next to a stream of water coming from the tap. The aim is to investigate how charge (electrons) can transfer between objects and how that can affect either objects behaviour or cause an effect.
Your hypothesis
What do you think will happen when you rub the balloon on your head? Write this down.
What will happen when you hold the balloon next to the stream of water? Write this down. Did you notice any other weird/unexpected effects when you rubbed the balloon against your hair? Describe any effects that you observed. This is not really part of your hypothesis for this experiment, but it is often the unexpected or odd things that scientists notice that can sometimes lead to amazing new discoveries and knowledge.
Method
1. Rub the balloon through your hair back and forth around 20 times (30 seconds).
2. Turn on the water tap so only a thin stream of water is running.
3. Hold the balloon to the side of the water stream – about 1-2 cm from the stream and about 8-10 cm below the tap. Make sure the balloon does not touch the water. Make sure it is the side of the balloon that you rubbed on your head that you put near the water. Observe what happens.
4. Move the balloon slowly up and down the stream of water. Observe what happens.
5. Move the balloon around the stream of water. Observe what happens.
6. Have a play and test some other ideas out. Try the comb (use it to comb your hair), plastic or glass rod rubbed on a towel and hold these items near the water stream. What happens if you get the balloon wet, or you have wet hair when you rub the balloon on it? Write down what you do here, step-by-step.
Tips: If it does not work, turn down the tap a bit to reduce the pressure of the water stream or run the balloon through your hair a few more times.
Results
Describe what you observed after each of the tests in steps 3 to 6 above.
(Did you notice anything about your hair when you rubbed the balloon against it? Can you describe it here?)
Discussion
What do you think was happening here? What does your data tell you that might help you answer this and the following questions? Use your knowledge of how charges behave to help you. That is, that opposite charges attract and like charges repel.
Why did the water behave the way it did?
Why did you get different effects/outcomes when your hair or balloon were wet?
Why does the water not fly straight to the balloon and stick to it? (Think about gravity.)
How did your findings match your hypothesis? Can you describe why your hypothesis was correct or incorrect?
Extend your thinking
What if the balloon or your hair is wet? What difference does it make to how your hair and stream of water reacts and why?
Did your hair stick out and try to attach to the balloon as you pulled the balloon away from your head? This happens because after rubbing the balloon on your hair, the electrons from the atoms in your hair jumped across to the balloon to make the balloon negative. What can’t move is the atoms that make up your hair. What is left after the electrons transfer from your hair to the balloon is atoms with more protons than electrons, which means your hair has a positive charge. Now what is your positive hair going to want to do when it is near a negatively charged balloon? Remember opposite charges attract. Remember also that each of your hairs is now positive. What do like charges want to do? Each hair on your head is trying hard to get as far away from all the other hairs (positive and positive will repel) while also being attracted to the negative balloon. It is why your hair is also all puffy and sticky out.
More stuff to figure out.
- What would happen if you used a metal comb or rod to try and transfer the electrons to?
- What if you had a non-polar liquid coming out of your tap? Could you make that bend with your negatively charged balloon?
Teacher notes
Have you noticed your hair sticking out on a dry day? Have you ever given someone a shock after rubbing your feet against a carpet? This experiment will help you understand static electricity and you will use this power to bend water.
What is happening?
This is all about a tale of opposing forces, how opposites attract and symmetry.
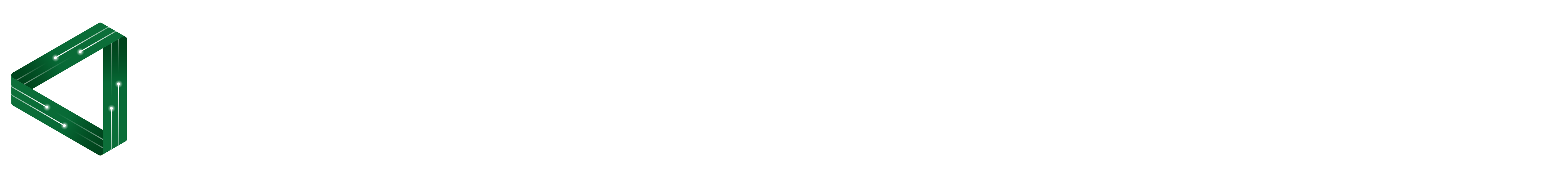
Water molecules are polar molecules. This means that the two hydrogen atoms and one oxygen atom that make up a water molecule form a shape that gives one end of the molecule a slightly positive charge and the other a slightly negative charge. A technical explanation is that they have an asymmetric charge distribution. But water molecules are still neutral overall; it is just their shape means that one end is more positive, the other end more negative. See Figures 1 and 2 below.
Polarity of molecules describes how the electrical charge is distributed around the molecule. In non-polar molecules, charge is evenly distributed. In a polar molecule such as water, that charge is unevenly distributed so it has regions that are more negative and regions that are more positive. A non-polar molecule is neutral at any position or region on the molecule. More information here.
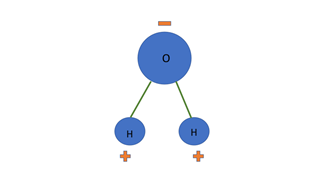
Figure 1. Water molecule showing its polar and asymmetrical shape that means one side is positive (where the hydrogen (H) atoms are) and the other side is negative (where the oxygen (O) atom is).
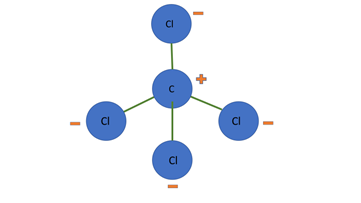
Figure 2. An image of a non-polar, symmetrical molecule where the charge (positive and negative) are evenly distributed around the molecule. The molecule is called Carbon Tetrachloride and the National Library of Medicine has a good animation that shows its symmetry.
The latex in a balloon is neutral – it has an equal number of positive and negative charges. But when you rub the balloon on your hair, electrons transfer from your hair to the balloon giving the balloon an overall negative charge. Electrons in some materials (eg, your hair) are loosely bound to their atoms. By rubbing the balloon onto your hair, the electrons on your hair leave their atoms and transfer to the balloon.
When you put the negatively charged balloon next to the stream of water, the water molecules in the stream will tend to flip so that their negative side is facing away from the balloon. Remember like charges repel – the negative charges (electrons) from the water and balloon will try to get as far apart from each other as they can. This means the positive sides of all the water molecules is now facing the negatively charged balloon. And what do opposite charges like to do? They are attracted to each other. The water molecules are attracted to the balloon, which is why you see the water bend toward the balloon. Gravity is also at work here. The gravitational force is pulling the water down, which is why the water stream does not fly straight out onto the balloon. See Figure 3 below.
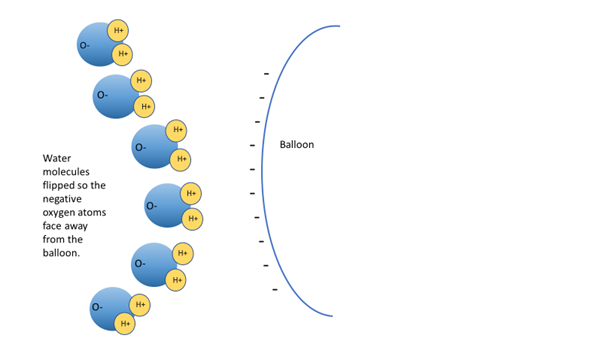
Figure 3. Water molecules in the presence of a negative charge (balloon) will orientate themselves so their negative side is facing away. The creates an overall positive charge closer to the balloon which is attracted to the negative charges (electrons) on the balloon.
What if the balloon or your hair is wet? What difference does it make to how your hair and stream of water reacts and why? Moisture prevents electrons moving around easily. If you fill your house with indoor plants this will reduce your ability to produce static electricity in the house because plants release water from their leaves and increase the humidity.
More stuff to figure out
- Look at a periodic table and count the number of protons and neutrons one oxygen atom and two hydrogen atoms have. There will be equal numbers of each which gives water (H2O) an overall neutral charge.
- Insulators such as plastic work best to hold onto the electrons transferred from your hair or cloth. The electrons in metals are mobile and when you transfer electrons from your hair or cloth to a metal rod, the free electrons in the metals will tend to run away from the transferred electrons from your hair (like charges repel). This makes it hard to build up an excess of electrons on a surface of metals. With insulators such as plastic the electron in the atoms are more tightly bound to their atoms, that is it is difficult for them to run away from the transferred electrons, so you can more easily get a build-up of electrons on a plastic balloon, comb or rod.
- If your liquid was non-polar it would not flip the same way as the polar water molecule could because the charge is equally distributed around the molecule. There is no part of the molecule that is more positive or negative. There would a much weaker (or zero) attractive force between the balloon (or plastic comb, rod, etc) and a non-polar liquid.
Learn more about conductors, insulators and electricity in the FLEET Schools resource
Acknowledgement: Thanks to FLEET Volunteer, Reza Asgari,for help with this activity.
Back to Home Science activities.