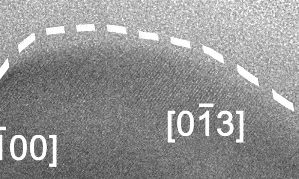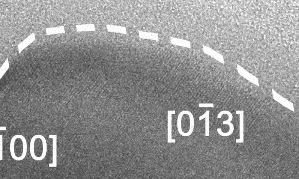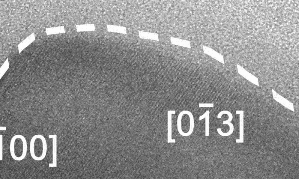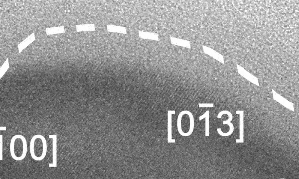
Liquid-solid boundary fluctuating back and forth, a phenomenon never seen before. Click here to watch full video with explanation on YouTube.
The boundary between solid metal and liquid metal can be much less ‘solid’ than we ever suspected.
RMIT researchers have discovered that the liquid-solid boundary can fluctuate back and forth, with metallic atoms near the surface breaking free from their crystal lattice.
Observing a metal-alloy mass solidifying in a sea of liquid metal, the team was able to observe a very interesting phenomenon, never seen before: the surface metal moves from a solid state into a liquid state, and back again.
In contrast to what is known as ‘pre melting’, this phenomenon occurred at unexpectedly low temperatures, far below the melting temperature of the solid metal (eg, 200°C below liquidus).
The phenomenon also occurs to a much greater depth than anticipated within the solid metal, up to 100 atoms in depth, and was seen to continue for several days.
In addition to being an exciting new fundamental discovery about the chemistry of solid and liquid metals, there is potential application ultimately where-ever metal alloys are utilised.
Observing metals on the move
In the experimental set-up, a solid (crystalline) metal-alloy mass forms in (or ‘precipitates’ from) a surrounding ocean of liquid metal, a common process in synthesising metal alloys.
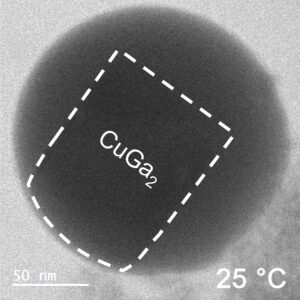
For example, a nugget of gallium-copper alloy might precipitate and grow in a sea of liquid gallium as it cools to room temperature, slightly below the melting temperature of gallium (30°C), but far below the melting temperature of the Cu-Ga alloy (256°C).
(The newly-observed fluctuating-surface phenomenon has occurred in all metal systems tested by the RMIT team, but is particularly well defined in the copper-gallium system.)
Despite the ubiquity of the liquid-metal alloying process, surprisingly little is known about the crucial surface chemistry of the process, due to the opaque nature of the liquid metal bath.
To solve this challenge, the team at RMIT directly imaged the surface phenomena of the gallium-copper mass using a Transmission Electron Microscope (TEM), which allows penetration of the liquid metal bath, and resolutions down to a nanometre scale.
At this scale, the surface of the solid alloy can be seen to be fluctuating between solid and liquid phase, at a rate of several times per second, and to a depth of around 10 nm, or 50-100 atoms.
“This fluctuation of the solid metal surface between solid and liquid phases was completely unexpected,” says lead author Caiden Parker, “because the entire system was being kept at close to room-temperature conditions.”
“The liquid gallium ocean was over 200°C colder than the melting point of the Cu-Ga alloy. There would have seemed no possible reason for its surface to keep reverting back to liquid form,” says Caiden, who is a FLEET PhD candidate at RMIT.
In the video, the crystalline Cu-Ga alloy is identifiable from the regular lattice structure, which appears as diagonal stripes. The surrounding grey area is liquid gallium and not empty space.
Escape and recapture: A molecular view of the fluctuating boundary
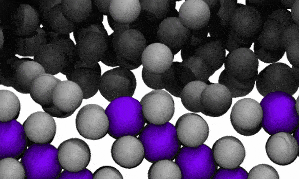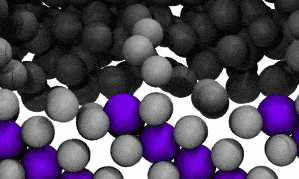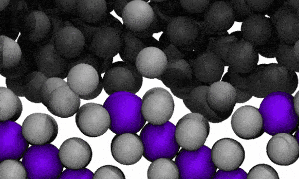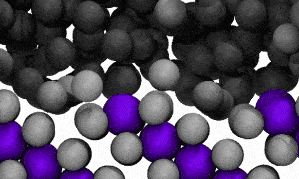
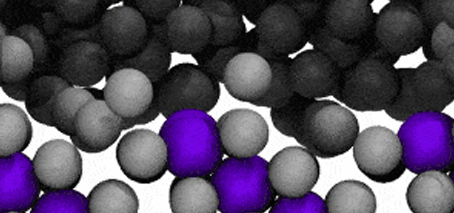
Modelling reveals Ga atoms breaking free of the crystal lattice. Click image for video.
“The outer layers of a solid metal alloy are surprisingly unstable when placed inside a liquid-metal environment, to the depth of several nanometres, fluctuating between crystalline and liquid states,” says team leader and corresponding author Prof Torben Daeneke (also at RMIT).
This crystal interface liquefaction, is observed at remarkably low temperatures (200°C below melting point of the solid), differentiating the observed liquefaction phenomenon from other processes such as surface pre-melting or conventional bulk melting.
The highly unstable crystal interface is observed in a variety of binary alloy systems and as such, the findings may impact the understanding of crystallisation and solidification processes in metallic systems and alloys more generally.
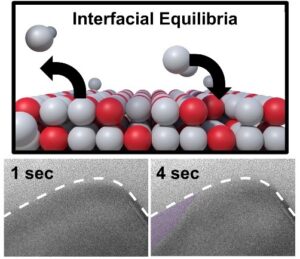
Liquid-solid boundary shrinks inwards with dissolution of bound gallium atoms, and then expands outwards with precipitation (recapture)
The crystal structure contains both ‘solute’ metal atoms (ie, copper) and ‘solvent’ metal atoms (gallium) thus forming a compound (CuGa2). The surface liquefaction process begins by losing some of the solvent metal atoms back into the surrounding liquid.
The researchers conducted molecular dynamic modelling in order to understand the observed surface fluidisation.
The modelling reveals that at the liquid-solid surface, some solvent (gallium) atoms will escape the solid structure due to that escape being energetically similar to staying in place.
Ie, a proportion of surface Ga atoms possess sufficient energy to escape the crystal lattice.
This ‘escape’ of atoms creates a vacancy at the surface, eventually creating an instability that leads to lattice collapse, causing the liquid-solid boundary to retreat inwards, into the solid.
Following this, the liquid becomes supersaturated in the solute (copper), forcing the surrounding liquid to re-bind with the crystal lattice. This causes the liquid-solid boundary to advance outwards again, back into the liquid.
The result is that the liquid-solid boundary oscillates backwards and forwards within a timespan of approximately half a second.
In the video of the molecular model, gallium atoms are represented in two colours: grey spheres represent gallium atoms that begin the modelled period being bound in the CuGa2 crystal lattice. Dark-grey spheres represent gallium atoms that begin the modelled period as moving freely in the surrounding liquid ocean.
The video shows a fraction of a nanosecond during the first phase of the process, when boundary shifts inwards as crystal-bound atoms escape to join the surrounding liquid.
As the model runs, grey atoms (ie, initially bound gallium atoms) escape the crystal lattice to float off into an ocean of dark grey (the surrounding liquid gallium). After a short while (a few hundred picoseconds), the purple atoms (ie, copper atoms) also begin to dislodge from the lattice.
Opportunities for further research and exciting future applications
“We hope this discovery will open new understanding of how metals behave, for creating new research opportunities, application in new alloy processes, solders, and improved additive manufacturing (3D printing) processes.
Crystallisation of alloys from a molten state is a fundamental metallurgic process, and the authors believe that the solid-liquid fluctuation of the crystal surface will be occurring every time crystallisation occurs.
“That’s why this is so exciting” says Torben. “The alloying process is so widespread, and so important in creating the materials that support modern industry….”
“Yet, no-one knew this was happening.”
“Now that we have discovered this fluctuation happening at the surface of solid alloys as they form, other metal chemistry researchers are going to want to explore this further.”
And with further improved fundamental understanding of the process of alloy crystallisation, it’s highly likely this newly discovered phenomenon will find an application.
The process of solidification in the synthesis of metal alloys is crucial, dictating the final physical, chemical and mechanical properties, all profoundly impacted by the final crystalline structure, size and shape.
“We can’t know yet what applications this might ultimately lead to,” says Caiden. “We don’t know whether someone will use this new understanding to synthesise improved alloys, or to reduce energy-use in alloy creation, or… who knows what!”
“That’s why fundamental science is so cool!”
The study
“Spontaneous Liquefaction of Solid Metal–Liquid Metal Interfaces in Colloidal Binary Alloys” was published in Advanced Science in April 2024. DOI: 10.1002/advs.202400147
As well as support from the Australian Research Council, facilities and equipment was made possible by RMIT University, and the RMIT Microscopy and Microanalysis Facility (RMMF).
The RMIT-led collaboration also included researchers from Monash University, UNSW, University of Sydney, University of Warwick and the ARC Centre of Excellence in Exciton Science.
More information
- Contact Prof Torben Daeneke (RMIT) torben.daeneke@rmit.edu.au
- Watch Future solutions to computational energy use
- Connect @FLEETCentre