Crystals grown in liquid-metal solvent show intricate, symmetrical metallic structures with a startling resemblance to snowflakes… plus potential applications in catalytic materials for producing hydrogen from organic fuels.
Liquid metals are enigmatic metallic solvents.
A new UNSW-led study of metallic crystals growing in a liquid metal solvent finds similarities and differences between liquid-metal solvents and more-familiar crystal-growth environments (such as water or the atmosphere) in which snowflakes or crystals of dissolved substances form.
We can dissolve a large amount of sugar in water at high temperatures. But as the water (the solvent) cools down, it becomes oversaturated and can dissolve less and less sugar, and so the excess amount of sugar that cannot stay dissolved in the solvent appears as crystals – first as numerous small crystallites, and then those sugar crystals grow larger and larger with time.
The new work reveals that a similar crystal formation phenomenon is also seen in liquid-metal solvents, although with significant differences.
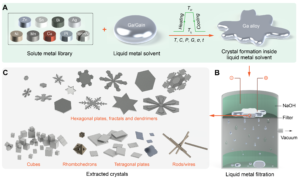
Liquid metals can directly dissolve many metallic elements. And just as with dissolution in water, the excess amounts of these solute elements make small crystals inside the liquid metal solvents. Then they grow into larger crystals, just like the sugar crystals in water.
However, there is an important difference between water and liquid metals: Liquid metal interacts with metallic surfaces of the crystals very strongly to form preferential growth directions and therefore peculiar crystals shapes, and such interactions are specifically governed by the metallic properties of the solvent and solute.
These are ‘metallic’ interactions, in comparison with other interactions that take place in water.
In liquid metals, we can form metallic crystals of different forms and shapes. We can even dissolve many metallic element types at the same time.
One challenge is the high surface tension of liquid metals. It is easy to make metallic crystals inside liquid metals, but their high surface tension makes it difficult to take them out. The new study demonstrated that by applying a voltage this problematic surface tension can be reduced, and the synthesised crystals can then be removed intact from the liquid metal solvent.
“There is an analogy to the characters in the ‘Transformers’ movies,” says lead author Dr Jianbo Tang (UNSW).
“In the movies, all the creatures are made from metals on their mother planet ‘’Cybertron’, where everything is also made of metals. So the main solvent in this planet should also be a liquid metal rather than water.”
The scientists were struck by the physical appearance of the crystals.
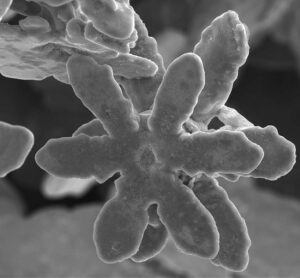
“We showed how beautiful metallic snowflakes of different crystal structures can be made inside liquid metals,” says corresponding author Prof Kourosh Kalantar-zadeh. “The similarities of morphology and growth between these metallic entities and natural snowflakes were amazing,” says Kourosh (UNSW, now University of Sydney).
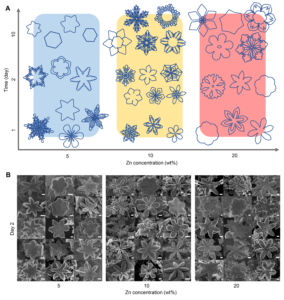
 In nature, snowflakes grow into fascinating, diverse, symmetrical six-branched shapes. In a liquid metal solvent, when zinc dissolves and then crystallises in liquid gallium, the resulting zinc crystals show high structural diversity and symmetry, and striking similarities to snowflakes.
In nature, snowflakes grow into fascinating, diverse, symmetrical six-branched shapes. In a liquid metal solvent, when zinc dissolves and then crystallises in liquid gallium, the resulting zinc crystals show high structural diversity and symmetry, and striking similarities to snowflakes.
The strategy offers general routes for creating highly crystalline, shape-controlled metallic or multi metallic fine structures from liquid metal solvents. In their future work, the team will move on to application-oriented metallic crystal synthesis and their structural fine tuning.
The study
Liquid metal synthesis solvents for metallic crystals was published in Science in December 8, 2022. (DOI: 10.1126/science.abm2731)
The work represents a collaboration between FLEET researchers at UNSW Sydney with colleagues at ANU and RMIT, and partner organisation the MacDiarmid Institute (NZ).
As well as funding by the Australian Research Council the authors acknowledge the Royal Society Te Apārangi’s Marsden Fund (NZ), New Zealand eScience Infrastructure (NeSI) high performance computing facilities and UNSW Tyree Micro CT Imaging Facility.
More information
- Contact Prof Kourosh Kalantar-zadeh (UNSW, University of Sydney) k.kalantar-zadeh@unsw.edu.au
- Contact Dr Jianbo Tang (UNSW) jianbo.tang@unsw.edu.au
- Contact A/Prof Torben Daeneke (RMIT) torben.daeneke@rmit.edu.au
- Contact Prof Nicola Gaston (MacDiarmid Institute) n.gaston@auckland.ac.nz