Evaluating student knowledge and learnings on electricity
A cooperative FLEET project with Ashburton Primary School in SE Melbourne is teaching students to think about atoms, electricity, conductors and insulators.
A pilot workshop with eighty-five students tested success in developing year-5 and 6s’ understanding of these areas, enabling critical thinking about applications of electricity and the value of research such as FLEET’s.
The pilot workshop had two broad aims:
- Building students’ understanding of atomic structure and electricity
- Promoting critical thinking about electricity, society’s application of it, and more broadly the value of FLEET research.
Evaluation built into the pilot indicated those objectives were met, but crucial to student understanding was their own investigation, examining a periodic table, building a model atom, and building different electrical circuits.
These hands-on investigations by the students helped build an understanding of how electricity works, the structure of the atom and role of charged particles (electrons and protons), the difference between conductors and insulator, and the nature and role of resistance.
The pilot, which aimed to promote critical thinking about electricity, society’s applications of electricity, and the value of research, was conducted online over two 1.5-hour sessions, with COVID restrictions preventing an in-person event. The use of Padlets to answer questions and share drawings allowed pre- and post-evaluation.
Thinking critically about electricity Students were asked to consider how electricity has changed the world and imagine how they would feel if we did not have electricity: “Without electricity, it would be hard to communicate to family that don’t live near you.” “There would be no toasters no baths no lights no iPads or PlayStation.” Coping with COVID without electricity would be bad: “We would feel sad and lonely in lockdown without power.”
While most such ideas were negative, some students considered how life without electricity might have positive effects, including getting more freedom, seeing more of their family, and motivating innovation: “…I would feel more healthy because I am not always looking at a screen.” “We will probably be innovative and we will invent new ways to live well without electricity.”

The ‘layered’ nucleus: several students considered neutrons and protons to occupy distinct positions within an atom’s centre
Introducing the atom and the power of the periodic table Students were asked to draw an atom, both before and after an in-class discussion and hands-on exercise around atomic structure.
Comparing student’s drawings of atoms before and after in-depth examination and construction of an atom model showed significant improvement, as would be expected.
Student’s initial ideas about atoms were often an indistinct blob, with no distinct nucleus. In some cases there were ‘hints’ of electrons, perhaps influenced by the introduction of electrons in class. Students also considered ‘an atom was an atom’. Ie, that all atoms were the same.
What really enabled them to conceptualize the atom was using the periodic table to select which atom to construct a model from using lollies.
The lolly model exercise not only allowed conceptualisation of atomic structure. It also provoked realisation that atoms come with different sizes and properties to make up all the different elements of the universe.
There was a realization that the type of atoms determines what they can feel, see, touch or smell: Students commented they’d learned that there are different types of atoms, and that “the different atoms are determined by the number of protons, neutrons and electrons.”
“The power of the periodic table as a learning tool in this context was unexpected!” says FLEET outreach coordinator Jason Major.

Lolly models: students built physical models to reinforce the numbers and positions of each of the 3 atomic components.
Following discussions and hands-on exercise building the ‘lolly model’ atom, students’ second drawings of atoms showed a significant improvement: a clearer central nucleus, surrounded by electrons in the next orbit.
Although the drawn nuclei often contained internal ‘layers’ of neutrons and protons, most students drew a relatively correct model, with a nucleus containing protons and neutrons, and electrons surrounding the nucleus. Students correctly labelled each of the protons, neutrons and electrons and had the appropriate number of each for their chosen element.
Building better circuits Building on their new knowledge of atoms and charged particles, students examined circuits, electrical conductors and insulators, and resistance.
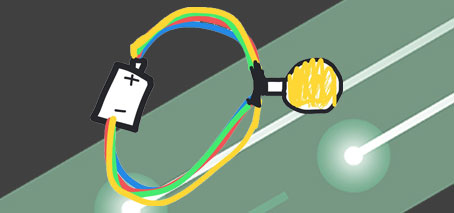
After an initial brainstorming session, students drew an electrical circuit. Similar to the atom drawing, this established a baseline understanding to help evaluate students’ learning.
Again, students would return to this drawing exercise after more investigation. In this case, an in-depth discussion, and, utilising the power of hands-on investigation, constructing circuits themselves from batteries, wires, light globes and electric motors, or using lead pencils to construct graphite circuits.
Students learned the basics of how to construct a functioning circuit, including practical considerations “When you power something up, the alligator clips get hot.”
Students had gained some understanding of the role of charged particles; and that electrons had to flow to generate charge: “Circuits need a negative and positive to work.” Students understood that resistance had a role in preventing electron flow and therefore electricity and there was insufficient flow of charge to operate two devices efficiently: “You can’t put two buzzers in a line” [series]. Students developed some understanding of the difference between parallel and series circuits.
Students began to conceptualize the role of the battery and voltage and the energy or force to push electrons through a circuit: I learned how the amount of energy affects how much the electricity works.” “High voltage refers to the power that an energy source provides.”
More than half the students had an incorrect concept of a circuit at the beginning of the lesson. However following in-class discussion and hands-on exercises, nearly all students managed to draw a correct circuit. Most understood which way electrons flowed through a circuit and the charged particles’ role in the flow of charge and electricity: “Electrons only produce electricity when moving.”
A few students then managed to set up draw a circuit in series.
Students struggled to conceptualize zero resistance. We asked students, What effect would we have if we generate a flow of charge – electricity – without resistance? Answers suggested this would be terrible: “Scary,” “Dead,” “The electricity would rush into your body and kill you”. This intriguing line of reasoning appeared to come from the idea that zero resistance would cause lethal levels of power in circuits.

Base case understanding: Less than half the students managed a correct circuit on their first attempt, with the need for multiple wires (or in other cases, not not enough wires) being common misunderstandings.
Considering the value of research As an example to get students thinking about societal value of science, we talked about FLEET’s research mission to develop low-energy electronics, within the context of the computational and energy requirement of digital technologies.
Students thought critically about the value of such research, with a high focus among students of societal challenges such as sustainability, climate change and making the world better: “We would waste less electricity” and “It would help reduce climate change.” They became aware of the value of reducing energy consumption and the value of having energy-efficient technologies to help achieve that goal.
Students reinforced their learning from Session 1 by thinking critically about the technologies they use and the consequences of this use.
Recalling the motivation of FLEET research: “10-20% of energy in Australia is lost through resistance.” “FLEET are looking to make power faster and more efficient.”
Many thanks to Joel Parsons and Courtney Simon from Ashburton Primary for their expert advice and invaluable contribution to the resource, for organizing materials for the pilot and for managing the students during the two sessions. The project was facilitated by the CSIRO STEM Professionals in Schools Program.