Injecting calcium into graphene creates a superconductor, but where does the calcium actually end up?
Adding calcium to graphene creates an extremely-promising superconductor, but where does the calcium go?
Adding calcium to a composite graphene-substrate structure creates a high transition-temperature (Tc) superconductor.
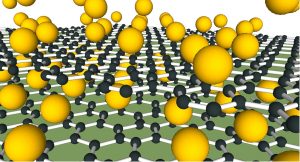
In a new study, an Australian-led team has for the first time confirmed what actually happens to those calcium atoms: surprising everyone, the calcium goes underneath both the upper graphene sheet and a lower ‘buffer’ sheet, ‘floating’ the graphene on a bed of calcium atoms.
Superconducting calcium-injected graphene holds great promise for energy-efficient electronics and transparent electronics.
Studying calcium-doped graphene: Throwing off the duvet
Graphene’s properties can be fine-tuned by injection of another material (a process known as ‘intercalation’) either underneath the graphene, or between two graphene sheets.
This injection of foreign atoms or molecules alters the electronic properties of the graphene by either increasing its conductance, decreasing interactions with the substrate, or both.
Injecting calcium into graphite creates a composite material (calcium-intercalated graphite, CaC6) with a relatively ‘high’ superconducting transition temperature (Tc). In this case, the calcium atoms ultimately reside between graphene sheets.
Injecting calcium into graphene on a silicon-carbide substrate also creates a high-Tc superconductor, and we always thought we knew where the calcium went in this case too…
Graphene on silicon-carbide has two layers of carbon atoms: one graphene layer on top of another ‘buffer layer’: a carbon layer (graphene-like in structure) that forms between the graphene and the silicon-carbide substrate during synthesis, and is non-conducting due to being partially bonded to the substrate surface.

Research was carried out in the New Horizon labs, Monash University, as well as the Australian Synchrotron
“Imagine the silicon carbide is like a mattress with a fitted sheet (the buffer layer bonded to it) and a flat sheet (the graphene),” explains lead author Jimmy Kotsakidis.
Conventional wisdom held that calcium should inject between the two carbon layers (between two sheets), similar to injection between the graphene layers in graphite. Surprisingly, the Monash University-led team found that when injected, the calcium atoms’ final destination location instead lies between buffer layer and the underlying silicon-carbide substrate (between the fitted sheet and the mattress!).
“It was quite a surprise to us when we realised that the calcium was bonding to the silicon surface of the substrate, it really went against what we thought would happen”, explains Kotsakidis.
Upon injection, the calcium breaks the bonds between the buffer layer and substrate surface, thus, causing the buffer layer to ‘float’ above the substrate, creating a new, quasi-freestanding bilayer graphene structure (Ca-QFSBLG).
This result was unanticipated, with extensive previous studies not considering calcium intercalation underneath the buffer layer. The study thus resolves long-standing confusion and controversy regarding the position of the intercalated calcium.
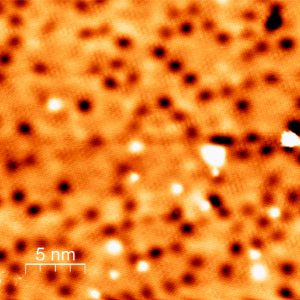
Measurements by STM (shown), XPS and LEED pinpointed the location of the calcium near the SiC surface
X-ray photoelectron spectroscopy (XPS) measurements at the Australian Synchrotron were able to pinpoint the location of the calcium near to the silicon carbide surface
Results were also supported by low-energy electron diffraction (LEED), and scanning tunnelling microscopy (STM) measurements, and by modelling using density functional theory (DFT).
And magnesium too…
With this information at hand, the Australian team also decided to investigate if magnesium–which is notoriously difficult to inject into the graphite structure –could be inserted (intercalated) into graphene on a silicon-carbide substrate.
To the researchers’ surprise, magnesium behaved remarkably similarly to calcium, and also injected between the graphene and substrate, again ‘floating’ the graphene.
Both magnesium- and calcium-intercalated graphene n-type doped the graphene, and resulted in a low workfunction graphene, an attractive aspect when using graphene as a conducting electrical contact for other materials.
But unlike calcium, magnesium-intercalated graphene remained stable in ambient atmosphere for at least 6 hours, overcoming a major technical hurdle for alkali and alkaline earth intercalated graphene.
“The fact that Mg-QFSBLG is a low workfunction material and n-type dopes the graphene while remaining quite stable in ambient atmosphere is a huge step in the right direction for implementing these novel intercalated materials in technological applications,” explains co-author Prof Michael Fuhrer.
“Magnesium-intercalated graphene could be a stepping stone towards discovery of other similarly stable intercalants.”
The study
The paper Freestanding n‑Doped Graphene via Intercalation of Calcium and Magnesium into the Buffer Layer−SiC(0001) Interface was published in Chemistry of Materials in July 2020 (DOI 10.1021/acs.chemmater.0c01729).
As well as the Australian Research Council (Laureate Fellowship and Centres of Excellence program), the authors acknowledge support of the Australian Government Research Training Program, Monash Centre for Atomically Thin Materials (MCATM), Ministerio de Ciencia Innovatión y Universidades, Comunidad de Madrid, and the US Naval Research Laboratory.
Computational support came from the Monash Campus Cluster, NCI computational facility and Pawsey Supercomputing Facility, and research was undertaken in part at ANSTO’s Australian Synchrotron.
More information
- Contact Jimmy C. Kotsakidis (Monash University) jckot1@student.monash.edu
- Contact Prof Michael Fuhrer (Monash University) Michael.Fuhrer@monash.edu
- Contact Dr Amadeo L. Vázquez de Parga (Universidad Autónoma de Madrid) al.vazquezdeparga@uam.es